

When an excitation energy is 500 cm −1, then about 8.9 percent of the molecules are thermally excited at room temperature. (The reciprocal centimeter is an energy unit that is commonly used in infrared spectroscopy 1 cm −1 corresponds to 1.239 84 ×10 −4 eV). At 298 K (25 ☌), typical values for the Boltzmann factor β are: We inspect the Boltzmann factor β ≡ exp(− Δ E / kT), where Δ E is the excitation energy of the vibrational mode, k the Boltzmann constant and T the absolute temperature. To get a feeling for the probability that the vibration of molecule may be thermally excited, At higher temperatures the vibrational modes may be thermally excited (in a classical interpretation one expresses this by stating that "the molecules will vibrate faster"), but they oscillate still around the recognizable geometry of the molecule. At absolute zero all atoms are in their vibrational ground state and show zero point quantum mechanical motion, so that the wavefunction of a single vibrational mode is not a sharp peak, but an exponential of finite width (the wavefunction for n = 0 depicted in the article on the quantum harmonic oscillator). The molecular vibrations are harmonic (at least to good approximation), and the atoms oscillate about their equilibrium positions, even at the absolute zero of temperature. (To some extent rotation influences the geometry via Coriolis forces and centrifugal distortion, but this is negligible for the present discussion.) In addition to translation and rotation, a third type of motion is molecular vibration, which corresponds to internal motions of the atoms such as bond stretching and bond angle variation. The overall (external) quantum mechanical motions translation and rotation hardly change the geometry of the molecule. Since the motions of the atoms in a molecule are determined by quantum mechanics, "motion" must be defined in a quantum mechanical way. The molecular geometry can be described by the positions of these atoms in space, evoking bond lengths of two joined atoms, bond angles of three connected atoms, and torsion angles ( dihedral angles) of three consecutive bonds. The position of each atom is determined by the nature of the chemical bonds by which it is connected to its neighboring atoms.

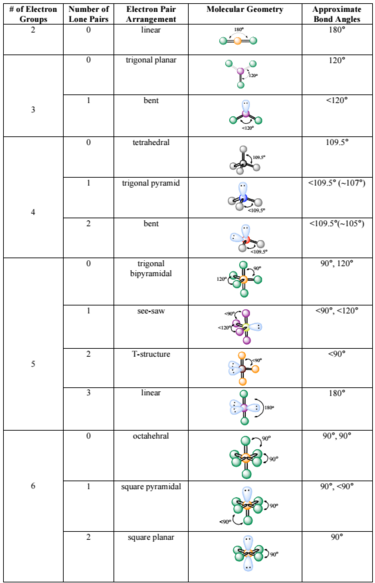
The molecular geometry can be different as a solid, in solution, and as a gas. Geometries can also be computed by ab initio quantum chemistry methods to high accuracy. Larger molecules often exist in multiple stable geometries ( conformational isomerism) that are close in energy on the potential energy surface. Molecular geometries are best determined at low temperature because at higher temperatures the molecular structure is averaged over more accessible geometries (see next section). NMR and FRET methods can be used to determine complementary information including relative distances, Īngles, and connectivity. Gas electron diffraction can be used for small molecules in the gas phase. X-ray crystallography, neutron diffraction and electron diffraction can give molecular structure for crystalline solids based on the distance between nuclei and concentration of electron density. IR, microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques. The molecular geometry can be determined by various spectroscopic methods and diffraction methods. they can be understood as approximately local and hence transferable properties. The angles between bonds that an atom forms depend only weakly on the rest of molecule, i.e. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism and biological activity. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. Geometry of the water molecule with values for O-H bond length and for H-O-H bond angle between two bonds


 0 kommentar(er)
0 kommentar(er)
